iso 8655 6 pipette calibration|iso 8655 2022 specifications : wholesaling Both pipette and tip manufacturers must disclose which pipette and tip combinations fulfill the ISO 8655 standards. 4. Pipette testing and calibration: Part 6, Part 7, and Part 8 Calibration sets the foundation for reliable and reproducible pipetting. Prior to the update, gravimetric measurement was the gold standard for calibration and testing. Our technicians carrying out the autoclave service and validation are fully trained, and City and Guilds certified to ensure the highest standards for every assignment. We attend to all makes .
{plog:ftitle_list}
Connaissez-vous les étapes indispensables à la stérilisation des aliments en conserve avec un autoclave ? TERRA Food-Tech® vous explique tout.
Information in the ISO 8655 includes standards for maximum permitted errors, guidelines for how to test and calibrate pipettes, reporting requirements to the end user, and how the end user .ISO 8655-6, ISO 8655-7 or ISO 8655-8. Any pipette so adjusted shall have clear, visible evidence that the initial adjustment has been modified. This information shall also be recorded. 5.4 Adjustment for other liquid properties Some pipettes are designed to have their factory pre-set adjustment altered by the user so that they willconditions, procedures, and equipment. For example pipette calibration in accordance with ISO 8655 is performed using a reliable measurement equipment, such as a high-quality balance, in a carefully controlled environment without any drafts or vibrations. ISO 8655 sets guidelines for:-- Characteristics, features, and marking of pipettes - Both pipette and tip manufacturers must disclose which pipette and tip combinations fulfill the ISO 8655 standards. 4. Pipette testing and calibration: Part 6, Part 7, and Part 8 Calibration sets the foundation for reliable and reproducible pipetting. Prior to the update, gravimetric measurement was the gold standard for calibration and testing.
Our ISO 8655-6 calibration services include a calibration certificate with extended uncertainty of measurement; for calibrations according to ISO 8655-7, we provide a test report without extended uncertainty. . ISO 8655-6:2022: The High Art of Pipette Calibration. Downloads: Calibration ISO 8655. Content. Brochures (7) Filter. Reset all .ISO 8655-6 Second edition 2022-04 Reference number ISO 8655-6:2022(E) . — calibration laboratories, test houses, users of the equipment and other bodies as a basis for . Pipettes ISO 8655-3, Piston-operated volumetric apparatus — Part 3: Burettes ISO 8655-4, Piston-operated volumetric apparatus — Part 4: Dilutors— general requirements for frequency of calibration, reporting measurement errors, exchangeable parts, metrological confirmation, routine testing, and maintenance and repair, suitability of . Pipettes ISO 8655-3, Piston-operated volumetric apparatus — Part 3: Burettes ISO 8655-4, Piston-operated volumetric apparatus — Part 4: Dilutors .Part 6 and Part 7 of the ISO 8655:2022 Part 6 and 7 of the ISO 8655:2022 specifies gravimetric measurement procedures for the determination of volume of piston-operated volumetric apparatus (POVA). Figure 1: Gravimetric Calibration of a Pipette on a Single Channel Balance Part 6 of the ISO 8655 describes the gravimetric reference
Note 1 to entry: When POVA are calibrated according to one of the procedures in ISO 8655-6, ISO 8655-7, or ISO 8655-8, the uncertainty of the mean delivered volume is usually estimated and reported. Note 2 to entry: When POVA are used in the laboratory the uncertainty in use of a single delivered volume can be estimated and is discussed further .The ISO 8655-6:2022 is an international standard for calibration and testing procedures of piston-operated volumetric apparatus like pipettes, burettes, dilutors, and dispensers, which must be performed at least once a year.calibration that the volume delivered is not within specification. Such user adjustment shall be made according to the manufacturer's instructions and by reference to the gravimetric test method specified in ISO 8655-6. Any piston pipette so adjusted shall have clear, visible evidence that the initial adjustment has been modified. This
ISO 8655 Compliant Pipette Calibration and Routine Testing Services, Systems, and Equipment. Request Info. Get a Quote. Request Quote or Info Call . To ensure reliable, accurate, traceable, and comparable results, all ISO 8655:2022 calibration methods (in 8655-6, 8655-7, and 8655-8) require a minimum of 10 repeated measurements per volume. .Our ISO 8655-6 calibration services include a calibration certificate with extended uncertainty of measurement; for calibrations according to ISO 8655-7, we provide a test report without extended uncertainty. . ISO 8655-6:2022: The High Art of Pipette Calibration. Downloads: Calibration ISO 8655. Content. Brochures (7) Filter. Reset all .ISO/IEC 17025 and ISO 8655 are among the most important standards that guide and define the pipette calibration industry. Each defines separate aspects of a quality calibration. . Together, these 6 requirements make ISO 8655 quite different and more “scientific” than the more process-oriented ISO 17025. In particular, .
ISO 8655:2022 and Sartorius Solutions for Pipette Calibration ISO 8655:2022 is an essential update for laboratories looking to ensure the accuracy and reliability of their volumetric . According to ISO 8655-6, it is imperative to use at least "Grade 3" water, as defined in ISO 3696, as a test liquid when checking pipettes. This is where the .ISO 8655 Compliant Pipette Calibration and Routine Testing Services, Systems, and Equipment. Request Info. Get a Quote. Request Quote or Info Call . To ensure reliable, accurate, traceable, and comparable results, all ISO 8655:2022 calibration methods (in 8655-6, 8655-7, and 8655-8) require a minimum of 10 repeated measurements per volume. .ISO 8655 is the official standard for piston-operated volumetric apparatuses, which includes pipettes, burettes, dilutors, dispensers, and manually operated precision laboratory syringes. An updated version of the standard came into force in May 2022 and included the following significant changes for pipettes:ISO 8655 Compliant Pipette Calibration and Routine Testing Services, Systems, and Equipment. Request Info. Get a Quote. Request Quote or Info Call . To ensure reliable, accurate, traceable, and comparable results, all ISO 8655:2022 calibration methods (in 8655-6, 8655-7, and 8655-8) require a minimum of 10 repeated measurements per volume. .

iso 8655 pipette standards
The test method described in ISO 8655-6 can be applied in every calibration procedure regardless of the ambient conditions. However, when the functionality of the equipment or the conformance to official acceptance limits are concerned, only the conditions mentioned in the standard are acceptable. . Results from multichannel pipette .ISO 8655 Compliant Pipette Calibration and Routine Testing Services, Systems, and Equipment. Request Info. Get a Quote. Request Quote or Info Call . To ensure reliable, accurate, traceable, and comparable results, all ISO 8655:2022 calibration methods (in 8655-6, 8655-7, and 8655-8) require a minimum of 10 repeated measurements per volume. . A calibration system was set up for calibrating multi-channel pipettes using the gravimetric method in accordance with the ISO 8655-6. The calibration system consisted of a display unit, a motor . We expect pipettes to accurately dispense liquid but after a while, pipettes will need calibration. The type of calibration your pipettes need may vary depending on usage, quality systems, and more. There are two primary types of ISO pipette calibration, which are known as ISO 8655 and ISO 17025.
ISO 8655:2022 defines two reference measurement procedures for pipette calibration: the gravimetric procedure (Part 6) and the photometric procedure (Part 8). Adhering to strict environmental conditions is crucial when using these reference procedures.
The ISO 8655-6:2022 is an international standard for calibration and testing procedures of piston-operated volumetric apparatus like pipettes, burettes, dilutors, and dispensers, which must be performed at least once a year.
Changes in the ISO 8655-6 with impact In April 2022, the revised ISO 8655-6:2022 for piston-operated volumetric apparatus was published. The following changes apply to test protocols under the new ISO 8655-6:2022: Number .ISO 8655: The International Standard for Pipettes ISO 8655 is the global standard that defines how pipettes should be manufactured and tested for accuracy. . considered one of the best methods for pipette calibration according. to ISO’s 2022 standard, whereas Part 7 provides several alternative methods. Also, Part 6 includes the following .
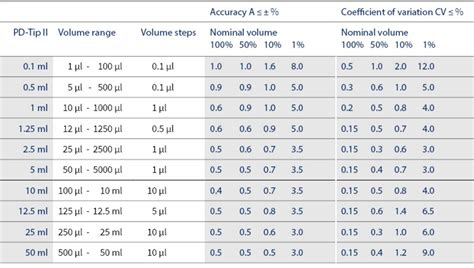
Minimum requirements of balances for micro pipette calibration as per ISO 8655-6 Table 3 Selected volume of the calibration liquid a V Resolution mg Repeatability and linearity mg Standard uncertainty of measurement mg . Document No. GD07 /11 Guidance Document on Calibration of Volume Copy No.ISO 8655 defines the useful volume range of a pipette as being from 10% of nominal to 100% of nominal. The most commonly used volume settings take after the ISO standard; the nominal volume (100%), 50% of nominal and the lower limit or .

leeb rebound hardness test method

iso 8655 pipette specifications
The AES Series vertical floor-standing autoclaves with top-loading access cover the fundamental needs for general labware sterilization in many industries, educational institutions
iso 8655 6 pipette calibration|iso 8655 2022 specifications